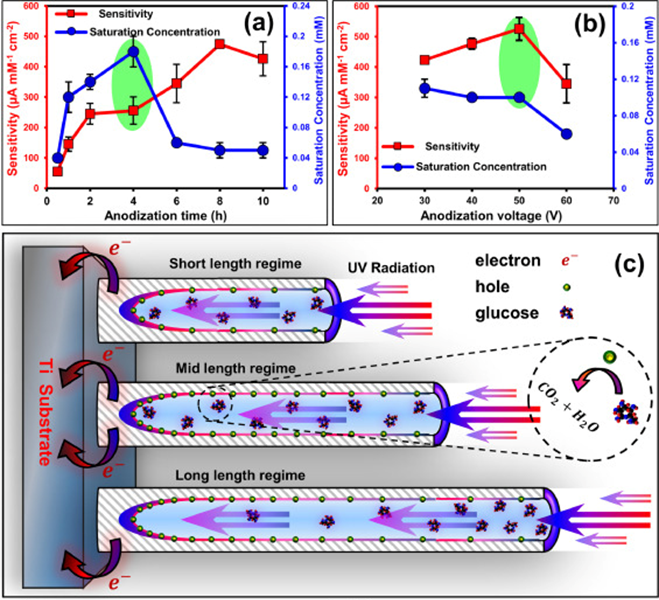
Bio-medical Application of Nanomaterials
Naimeh Naseri2022-08-09T14:26:27+00:00A particularly alarming issue in world health today is the rise and prevalence of antibiotic-resistant bacteria, which significantly increases death rates and costs of treatment; and a group of pathogens responsible for the majority of hospital acquired infections. On 27 February 2017, WHO published list of bacteria for which new antibiotics are urgently needed. Although the Gram-positive bacteria in the ESKAPE group, including the methicillin-resistant Staphylococcus aureus, have rightly drawn attention over the past decade, infections caused by the Gram-negative microbes have recently been recognized as a more critical healthcare issue. One the other hand, detecting bacteria and other pathogenic species as well as bio-analytes like anti-oxidants, glucose, etc. is also vital.
Sensitive and selective target capture, recognition, and signal transduction in detection of chemical and biological molecules is essential for fundamental biomedical studies, disease diagnosis, and drug screening. To achieve fast, sensitive, large-scale, and low-cost molecular analysis, a wide variety of detection technologies such as fluorescent, spectroscopic, electrical, magnetic and mechanical methods have been developed.
Generally, there are three layers of complexity that are interconnected and need to be considered carefully in the development of nanomaterials for use in biomedical applications: material characteristics; interactions with biological components and biological activity outcomes. To understand and follow antibacterial and/or sensing mechanisms of nanomaterials, it is critical to know how their properties are determinant in their final performances.
To know more about fundamentals and details of the subject, some useful review papers are published like:
1- Hegab et al. The controversial antibacterial activity of graphene-based materials. Carbon, 2016.
2- Shi et al. The Antibacterial Applications of Graphene and Its Derivatives, Small, 2016.
5- Bai et al. Titanium Dioxide Nanomaterials for Sensor Applications, Chemical Reviews, 2014.
Machine Assisted Approaches for Material Design and Device Fabrication
Naimeh Naseri2022-08-09T14:26:11+00:00Useful information on this subject can be found in informative published papers like:
5- Gaviria et al. Machine learning in photovoltaic systems: A review, Renewable Energy, 2022.
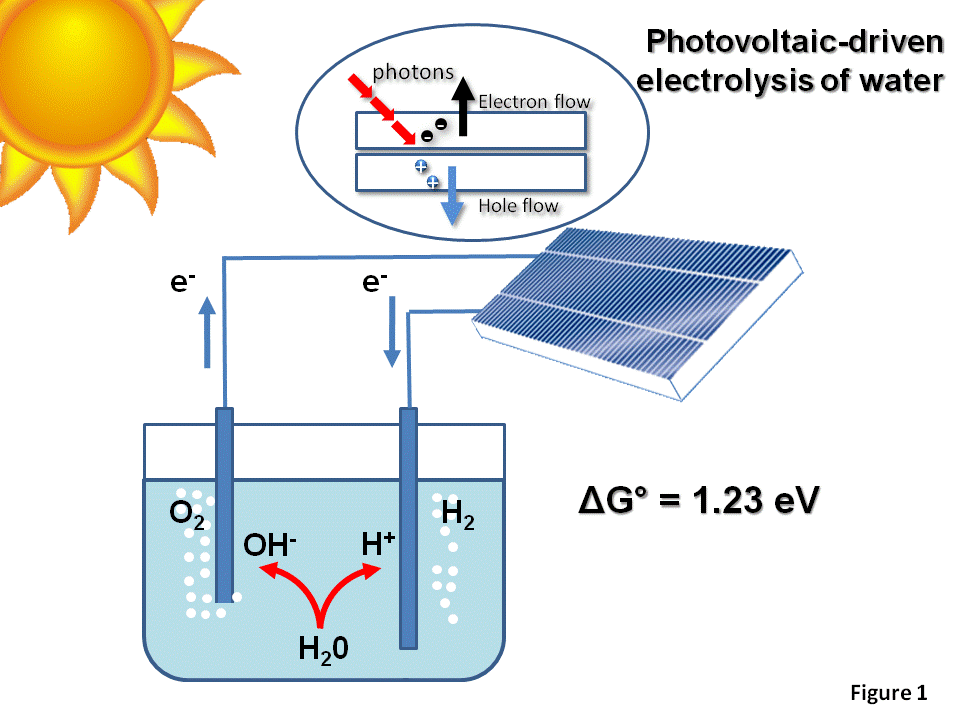
Earth Abundant Electro-catalysts for Red/Ox Reactions
Naimeh Naseri2022-08-09T13:56:50+00:00Considering the depletion of hydrocarbon reservoirs and adverse effects of their combustion, providing a renewable and environmentally friendly resource of energy is necessary for human societies. Photoelectrochemical (PEC) and electrochemical (EC) water splitting into O2 and H2 at the surface of a proper material (photoactive semiconductor or electrocatalysts) is a promising solution to produce solar fuel as a green and sustainable source of energy. In both these approaches, each half-reaction, oxygen evolution reaction (OER) and hydrogen evolution reaction (HER), must be optimized to achieve the highest efficiency.
The challenging step is the OER since its Gibbs free energy is 1.23 eV per electron while kinetic barriers (known as overpotential) may increase this amount up to ~2 eV. In order to reduce the overpotential, a proper electrocatalyst should be applied to facilitate surface hole transfer and raise reaction kinetics. In this context, materials employed as cocatalysts in PEC systems must be chosen among earth abundant and chemically stable compounds. According to extensive researches in this area, iridium and ruthenium-based co-catalysts are found to exhibit the lowest overpotential but not applicable due to their high cost and rarity. As proposed by well-known volcano plot, the most attracting candidates for oxygen evolution reaction are first row transition metals such as Co and Ni in the form of oxide/hydroxide nanostructures which are abundant with variety of low cost preparation methods. Recently, efficient electrocatalysts for HER are also proposed based on 2D MoS2 nanosheets. In the following review papers, basic information about parameters as well as recent trends can be found.
1- Thorarinsdottir et l. Self-healing oxygen evolution catalysts, Nature Communications, 2022.
2- Roger et al. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting, 2017.
3- Kanan et al. Cobalt–phosphate oxygen-evolving compound, 2009.
4- Chen et al. Recent Progress on Nickel‐Based Oxide/(Oxy)Hydroxide Electrocatalysts for the Oxygen Evolution Reaction, 2018.
5- Lyu et al. Noble‐Metal‐Free Electrocatalysts for Oxygen Evolution, 2018.
6- Guo et al. Phosphate‐Based Electrocatalysts for Water Splitting: Recent Progress, 2018.
7- Zhao et al. Heterostructures for Electrochemical Hydrogen Evolution Reaction: A Review, 2018.
8- Wang et al. Recent Progress on Layered Double Hydroxides and Their Derivatives for Electrocatalytic Water Splitting, 2018.
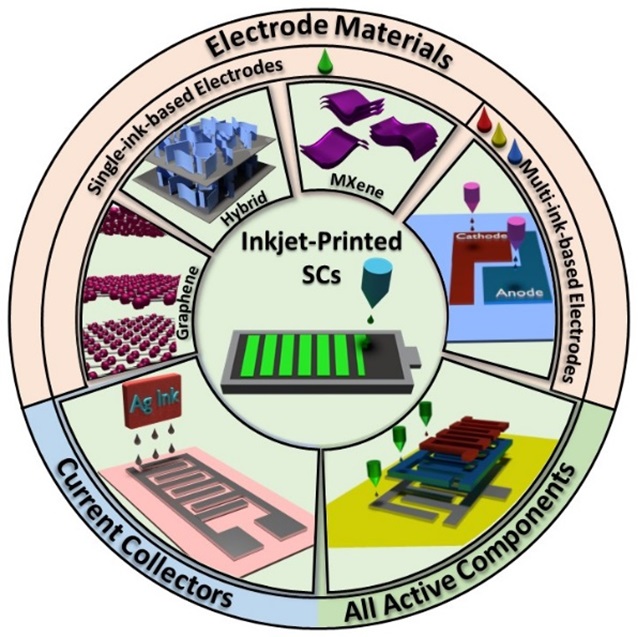
Additive Manufacturing/ Printing of Devices
Naimeh Naseri2022-08-09T13:56:35+00:00Today, due to emerging 4th industrial revolution (I 4.0), additive manufacturing methods for fabricating a variety of devices are very attractive. Besides different techniques like PBF, DIW, SLA, FDM, ink-jet printing (IJP) provides a powerful tool for the fabrication of functional devices with high accuracy. The operating principle of IJP technique is close to the direct ink writing (DIW) process. In these techniques, stable and homogeneous inks with desired rheological properties are formulated by dispersing active material in a liquid medium. Despite the similarities, the rheological properties of developed inks for each of these methods are different.
Here, some papers are listed as useful sources:
4- Chu et al. 3D printing‐enabled advanced electrode architecture Design, Carbon energy, 2020.
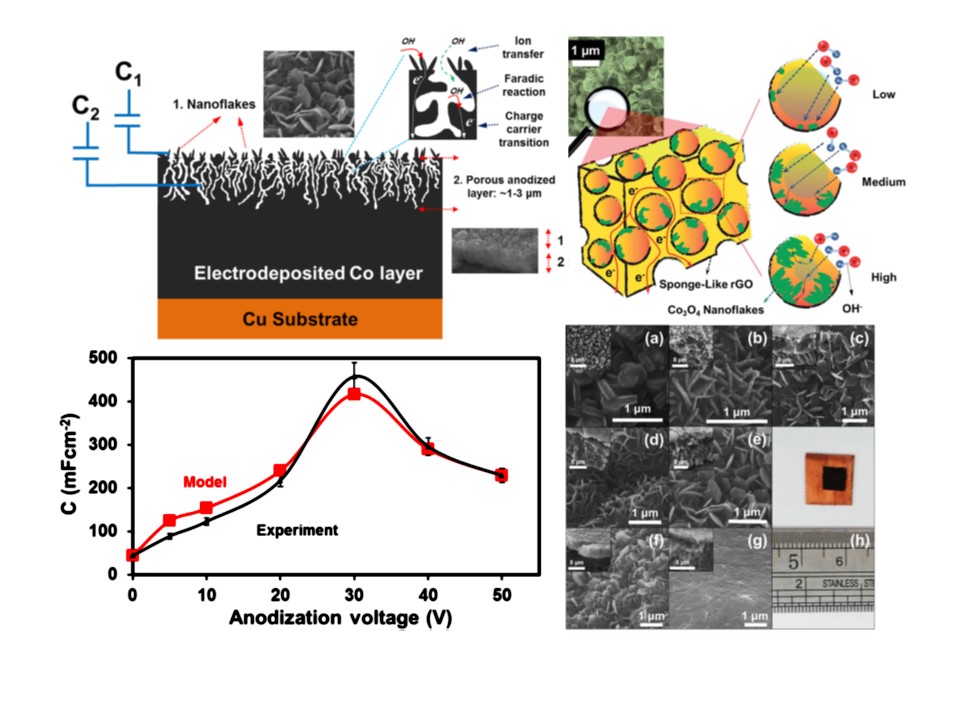
Nanomaterials for Energy Storage Applications
Naimeh Naseri2022-08-09T13:55:56+00:00Supercapacitor devices are well-known as their fast charge storage kinetics by the electric double-layer capacitance (EDLC) or EDLC-like mechanisms at the electrode/electrolyte interfaces. So far, research groups worldwide have been mostly worked on the electrodes consist of carbon-based (e.g. porous carbon, graphene, curved graphene nanosheets, graphene nanoribbons, carbide-derived carbon and etc.), two dimensional layered (e.g. Ti3C2Tx MXene) and transition metal oxide/sulfides/phosphates (e.g. MnO2, Co3O4 and etc.) materials. The former stores charge by the adsorption of electrolyte ions onto the surface of the electrode materials providing high power density. The two latter ones store charge by fast redox reactions presenting an intrinsic (such as MnO2) or extrinsic (such as LiCoO2 thin films) pseudocapacitive properties providing high energy density.
More useful information on this subject can be found in informative published papers like:
4- Gogotsi et al. Where Do Batteries End and Supercapacitors Begin? Science, 2014.
5- Brousse et al. To Be or Not To Be Pseudocapacitive? journal of the Electrochemical Society, 2015.
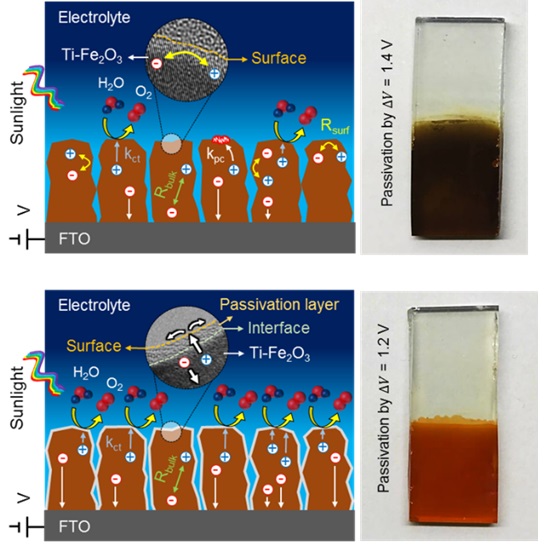
Solar Hydrogen Production from Water
Naimeh Naseri2022-08-09T13:55:31+00:00The alarming depletion rate of reserved fossil fuel associated with rapid increase in environmental pollution has caused an urgent need to develop efficient clean and renewable energy resources. In this regard, many different approaches have been followed up. The sun is a free, clean, sustainable and easy access energy source, and the solar produced hydrogen, which can be used in fuel cells to generate electricity or changed directly in combustion engines, makes no pollution except water. Hence, to obtain hydrogen as a clean energy carrier, the scenario of a renewable hydrogen economy has attracted much attention from researchers recently. Because of low operation temperature and strong synergies with contemporary researches in the field of photovoltaic and nanomaterials, photoelectrochemical (PEC) water splitting is an emerging technology for the future world hydrogen generation. The concept of this method is based on a semiconductor photoelectrode device which excites with sunlight irradiation, oxidizes/reduces H2O molecules by generated h+/e− pair and finally converted them to chemical energy (H2 gas). For efficient PEC reaction, the selected semiconductor photoanodes should exhibit chemical stability, suitable band edge positions for absorbing sunlight and also participating in water oxidation/reduction, high charge carrier mobility and also variety of low cost synthesis methods.
Photoelectrodes based on transition metal oxides, TMDCs or other emerging 2D materials have been extensively investigated in aqueous solution. Different approaches have been followed to prohibit charge recombination and improve visible response of these photoanodes. Many interesting reports and reviews are available in this context which present recent strategies and trends to improve solar hydrogen production systems in PEC, EC or PC approaches.
5- Viory et al. Low-dimensional catalysts for hydrogen evolution and CO2 reduction, 2018.
e
